Global Blood Therapeutics Fda
GBT today announced that the US. GBT CRSP NKTR SPPI 32 Comments.
 Long Term Results On Oxbryta Published In Journal
Long Term Results On Oxbryta Published In Journal
GBT today announced that six abstracts related to its sickle cell disease SCD programs will be presented at the European Hematology Association EHA 2021 Virtual Congress taking place online June 9-17 2021.
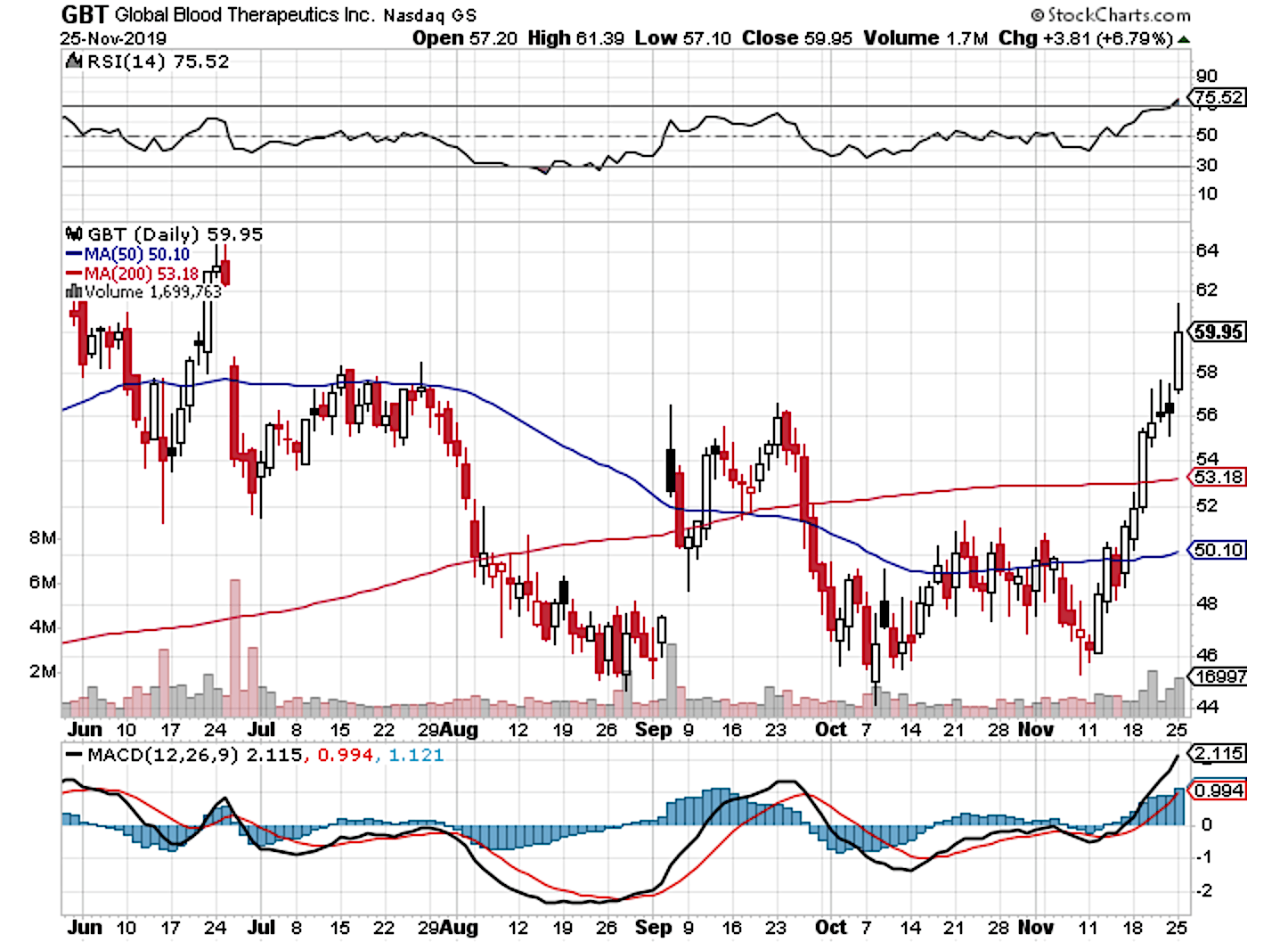
Global blood therapeutics fda. SOUTH SAN FRANCISCO Calif May 12 2021 GLOBE NEWSWIRE -- Global Blood Therapeutics Inc. The treatment will be priced at 10417 per month or around 125000 per year and will be sold under the brand name Oxbryta. Global Blood Therapeutics Inc stock is down 657 over the past week and gets a Neutral rating from InvestorsObservers Sentiment Indicator.
External links are being provided as a convenience and for informational purposes only. Food and Drug Administration said on Monday it approved a drug from Global Blood Therapeutics Inc to treat sickle cell disease in adults and children 12 years or older. SOUTH SAN FRANCISCO Calif Sept.
Find out what this means for you and get the rest of the rankings on GBT. Global Blood Therapeutics GBT Announces Upcoming Data Presentations at European Hematology Association EHA 2021 Virtual Congress. It also expects to soon submit for US.
Global Blood Therapeutics. 05 2019 GLOBE NEWSWIRE -- Global Blood Therapeutics Inc. However biotech investors might not.
Reuters - The US. Global Blood Therapeutics said that its on track to kick off two pivotal late-stage studies of another sickle-cell disease drug inclacumab by mid-2021. Side effects can also be reported to Global Blood Therapeutics at 1-833-428-4968 1-833-GBT-4YOU.
SOUTH SAN FRANCISCO Calif Nov. 25 2019 GLOBE NEWSWIRE -- Global Blood Therapeutics Inc. The FDA an agency within the US.
External links are being provided as a convenience and for informational purposes only. They do not constitute an endorsement or an approval by Global Blood Therapeutics. The FDA granted the approval of Oxbryta to Global Blood Therapeutics.
You are now leaving the Global Blood Therapeutics website You are currently leaving our site. Department of Health and Human Services protects the public health by assuring the safety. 20 2020 to make its decision.
The FDA accepted Global Bloods New Drug Application NDA for voxelotor and has until Feb. GBT today announced that the US. They do not constitute an endorsement or an approval by Global Blood Therapeutics.
This month the drugs developer Global Blood Therapeutics GBT in South San Francisco California revealed that FDA has launched a priority review of. Food and Drug Administration said on Monday it approved a drug from Global Blood Therapeutics Inc to treat sickle cell. Global Blood Therapeutics has its first drug approval and its one thatll bring a paradigm shift in how.
Global Blood Therapeutics Inc has a Neutral sentiment reading. In acquiring the two early-stage Sanofi drugs for sickle cell disease Global Blood Therapeutics aims to complement Oxbryta its FDA-approved treatment for. Global Blood Therapeutics NASDAQ.
What The Recent FDA Breakthrough Therapy Designation For Voxelotor Entails Jan. You are now leaving the Global Blood Therapeutics website You are currently leaving our site. Food and Drug Administration FDA has accepted for.
14 2018 221 AM ET Global Blood Therapeutics Inc. Reuters The US. GBT announced that it had received FDA approval for its drug Oxbryta voxelotor in treating adults.
Another day another early FDA nod. Corporate News FDA. Food and Drug Administration FDA has granted accelerated approval for Oxbryta voxelotor tablets for the treatment of sickle cell disease SCD in adults and children 12 years of age and older.
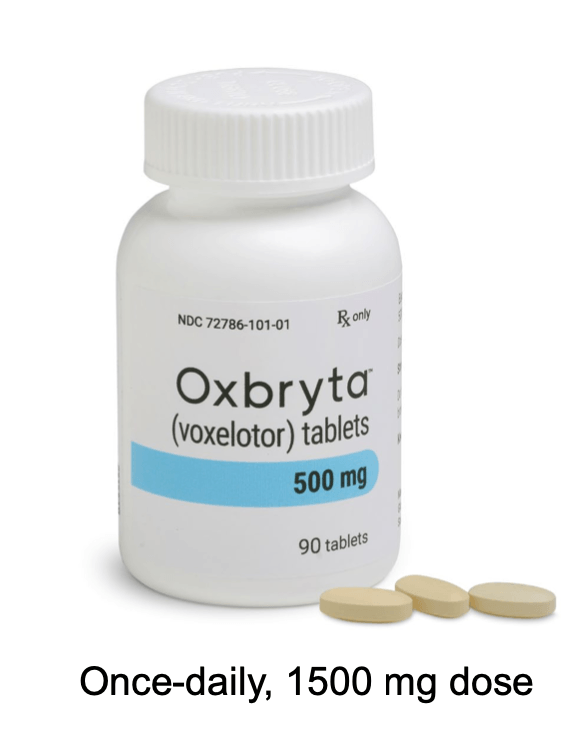
1 Oxbryta an oral therapy taken once.
 Global Blood Therapeutics Oxbryta Is Aimed For Success Nasdaq Gbt Seeking Alpha
Global Blood Therapeutics Oxbryta Is Aimed For Success Nasdaq Gbt Seeking Alpha
Gbt Expands Sickle Cell Disease Pipeline With Exclusive

 Gbt Announces Plans To Seek Regulatory Approval For Oxbryta Voxelotor To Treat Sickle Cell Patients In Europe Tif
Gbt Announces Plans To Seek Regulatory Approval For Oxbryta Voxelotor To Treat Sickle Cell Patients In Europe Tif
Global Blood Therapeutics Sickle Cell Disease Drug Hits The Mark In Phase Iii Trial Biospace
 New Sickle Cell Drugs From Novartis Gbt Need Big Discounts Icer Draft Fiercepharma
New Sickle Cell Drugs From Novartis Gbt Need Big Discounts Icer Draft Fiercepharma
 Eha 2019 Global Blood Faces Sickle Cell Questions Evaluate
Eha 2019 Global Blood Faces Sickle Cell Questions Evaluate
 Global Blood Therapeutics 40 Discount On A Billion Dollar Drug Nasdaq Gbt Seeking Alpha
Global Blood Therapeutics 40 Discount On A Billion Dollar Drug Nasdaq Gbt Seeking Alpha
 7 Global Blood Therapeutics Fiercebiotech
7 Global Blood Therapeutics Fiercebiotech
 Fda Agrees Accelerated Approval Pathway For Gbt S Voxelotor Onescdvoice
Fda Agrees Accelerated Approval Pathway For Gbt S Voxelotor Onescdvoice
 Forma Therapeutics Sickle Cell Disease Therapy Gets Us Fda Orphan Drug Status S P Global Market Intelligence
Forma Therapeutics Sickle Cell Disease Therapy Gets Us Fda Orphan Drug Status S P Global Market Intelligence
 Global Blood Therapeutics Oxbryta Is Aimed For Success Nasdaq Gbt Seeking Alpha
Global Blood Therapeutics Oxbryta Is Aimed For Success Nasdaq Gbt Seeking Alpha
 Dr Ted W Love Of Global Blood Therapeutics 5 Steps We Must Take To Truly Create An Inclusive Representative And Equitable Society By Alexandra Spirer Authority Magazine Medium
Dr Ted W Love Of Global Blood Therapeutics 5 Steps We Must Take To Truly Create An Inclusive Representative And Equitable Society By Alexandra Spirer Authority Magazine Medium
Fda Approves Oxbryta Voxelotor The First Medicine
 Global Blood Therapeutics 40 Discount On A Billion Dollar Drug Nasdaq Gbt Seeking Alpha
Global Blood Therapeutics 40 Discount On A Billion Dollar Drug Nasdaq Gbt Seeking Alpha
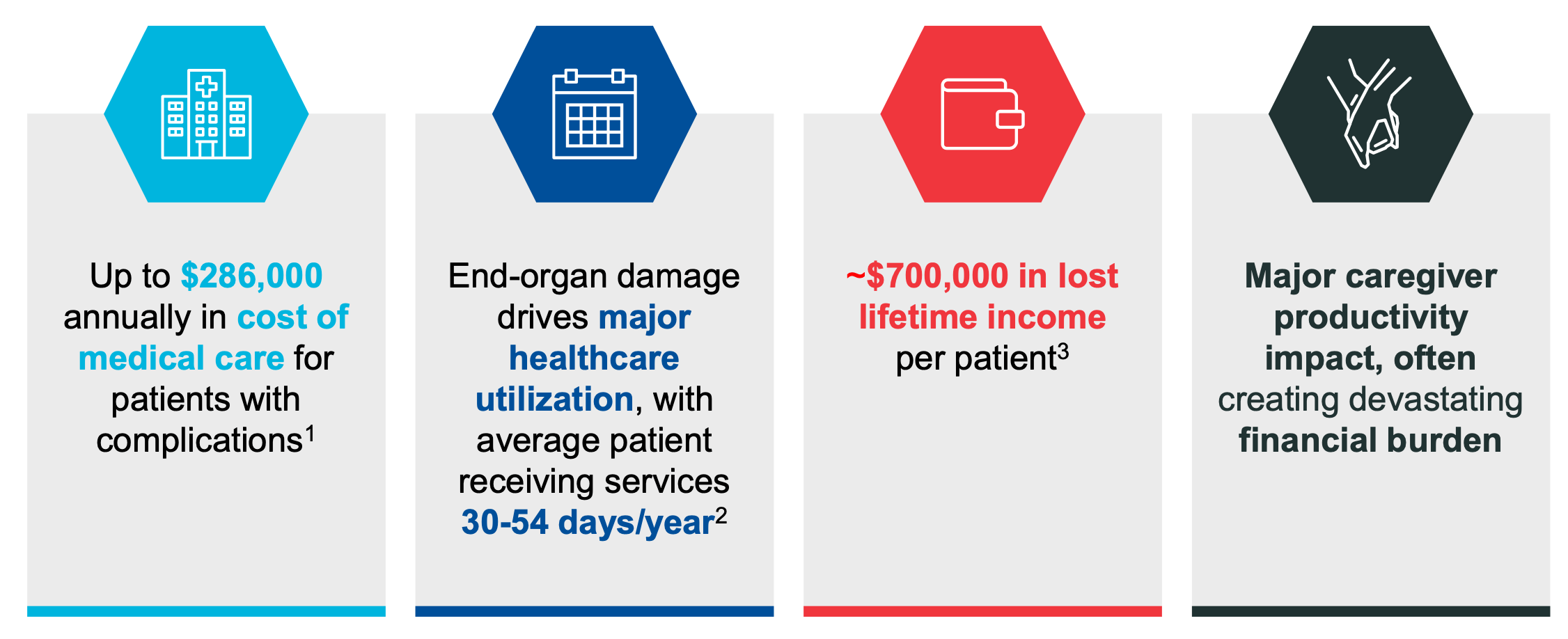 Global Blood Therapeutics 40 Discount On A Billion Dollar Drug Nasdaq Gbt Seeking Alpha
Global Blood Therapeutics 40 Discount On A Billion Dollar Drug Nasdaq Gbt Seeking Alpha
 Jonathan Sorof M D Global Blood Therapeutics
Jonathan Sorof M D Global Blood Therapeutics


Post a Comment for "Global Blood Therapeutics Fda"