Global Quality & Risk Management Manual
A key area is insuring a top management focus that is knowledgeable and understanding of the qualityauditing process across industry type. It is the policy of uOttawa to preserve the assets of the institution and protect the physical.
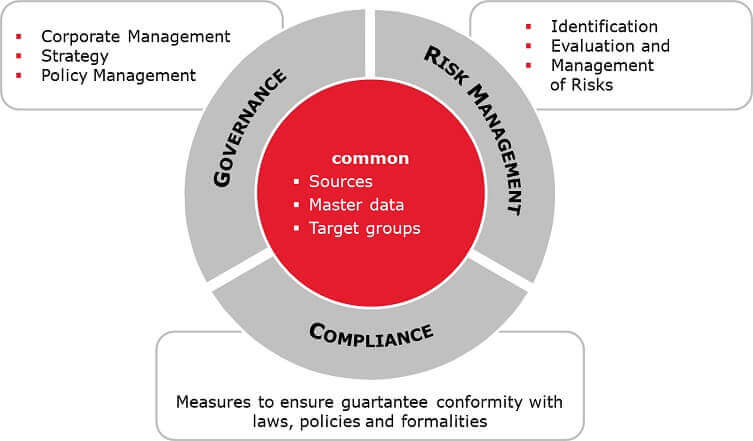 The Essential Guide To Governance Risk Management And Compliance Grc Tallyfy
The Essential Guide To Governance Risk Management And Compliance Grc Tallyfy
Quality Manual Template wwwiso9001helpcouk ISO 90012015 Quality Management System Document Ref.
Global quality & risk management manual. The procedures and policies that underpin our efforts in respect of these principles are set out in our Code of Conduct Global Marketing Risk Management GMRM and Global Quality and Risk Management Manual GQRM Manual. A model for quality risk management is outlined in the diagram Figure 1. The Quality Assessment Manual for the Internal Audit Activity published by the Internal Audit Foundation continues to be the principal methodology and set of practical tools recommended for evaluation of the QAIP in order to assess conformity to the Standards leading practices and equally important to reveal opportunities for enhancing the effectiveness and.
System of quality control and risk management. Risk Management Manual of Examination Policies Complete Manual - ZIP 10MB Current Year Updates. A Purpose of Manual This Risk Management Manual provides the framework to decrease the frequency of incidents and to also reduce the impact of incidents if they do occur while balancing this with taking risks.
They provide a framework for considering everything an organisation does how it is done and identifying ways. Risk management and quality improvement are not isolated processes. These are documented in the Global Quality Risk Management Manual GQRM Manual available to all member firms and their personnel on a web-based platform.
These policies and associated procedures are designed to assist member firms in complying with relevant professional standards regulatory and legal requirements and in issuing reports that are appropriate to the circumstances. The leadership will ensure that the quality management system and its context is demonstrated throughout promoting the use of risk based thinking to predict and prevent issues internally and externally review resources at. December 2020 Updates Only - ZIP November 2020 Updates Only - ZIP October 2020 Updates Only - ZIP August 2020 Updates Only - ZIP May 2020 Updates Only - ZIP.
April 2021 Updates Only - ZIP February 2021 Updates Only - ZIP Prior Year Updates. Review their quality budget and quality table of organization. The quality management system at the highest positions within the company.
Other models could be used. The process is a series of coordinated activities designed to detect and control risks. These drastic changes have gone from solely a doctors word to patient-centered care.
It is based on risk discussions conducted by the leadership teams of business units at the global level in alignment with their own strategic planning. The GQRMSG is responsible through the Quality Performance Review QPR the Risk Compliance Program RCP Global Compliance Reviews GCR and Area Quality and Risk Management Leaders ARLs for monitoring. The Global People Performance and Culture Manual informs the conduct to be adopted at each of the human resource management areas as an integral part of the process for analyze quality and risk management attending to the norms enacted by the local laws and also professional requirements in Brazil requirements of the Federal Accounting Council CFC IBRACON Institute of.
The means to ensure the effective planning operation and control of its processes. The Global People Performance and Culture Manual informs the conduct to be adopted at each of the human resource management areas as an integral part of the process for analyze quality and risk management attending to the norms enacted by the local laws and also professional requirements in Brazil requirements of the Federal Accounting Council CFC IBRACON Institute of Independent. July 2013 Developing a Commitment to Risk Management and Quality Improvement using EQuIPNational.
Held at least once every three years these reviews assess whether member firms comply. Page 17 of 51 6 Management System Planning 61 Addressing Risks Opportunities In order for our organization to have a successful quality management system we. Stephan Rönninger Pharma Medicines Technical Operations Global Quality Compliance F.
Hoffmann-La Roche Ltd. Quality risk management is a systematic process for the assessment control communication and review of risks to the quality of the drug medicinal product across the product lifecycle. Each member firm is responsible for conducting its own practice reviews under the guidance and oversight of Deloitte Global.
The Risk Resilience team introduced an innovative integrated enterprise risk management ERM approach. RISK AND QUALITY MANAGEMENT MANUAL 2 Risk and Quality Management Manual Over the years the health care industry has changed drastically. Current Global GMP Status and Trends With Focus on EU PICS JPMA Annual Meeting Tokyo Osaka September 2012 Dr-Ing.
For a list of global level quality specifications and procedures reference Quality Specification TEC-1017 Global Quality Management System Cross-Reference for Policies Specifications and Standards. ICH Q9 Quality Risk Management. These are included in KPMGs Global Quality Risk Management Manual GQRM Manual available to all KPMG partners and staff.
Determine their goals and who manages quality for ongoing training and education including for a continually updated Supplier Quality Manual. Because the risk landscape continues to be volatile uncertain and complexwith increasing demands from clients and greater scrutiny from regulators legislators and other governmental authoritiesDeloitte continues to focus relentlessly on quality and risk management QRM actively monitoring strengthening and improving its risk management processes and procedures and promoting a. Risk Management and Quality Improvement Handbook.
Patients are now afforded the opportunity to discuss the care a doctor suggests and make the final decision if they agree to what a doctor is ordering.
Medical Device Design Risk Management Basic Principles Wipro
Https Www Theglobalfund Org Media 7540 Financial Financialriskmanagement Guidelines En Pdf U 636784020850000000
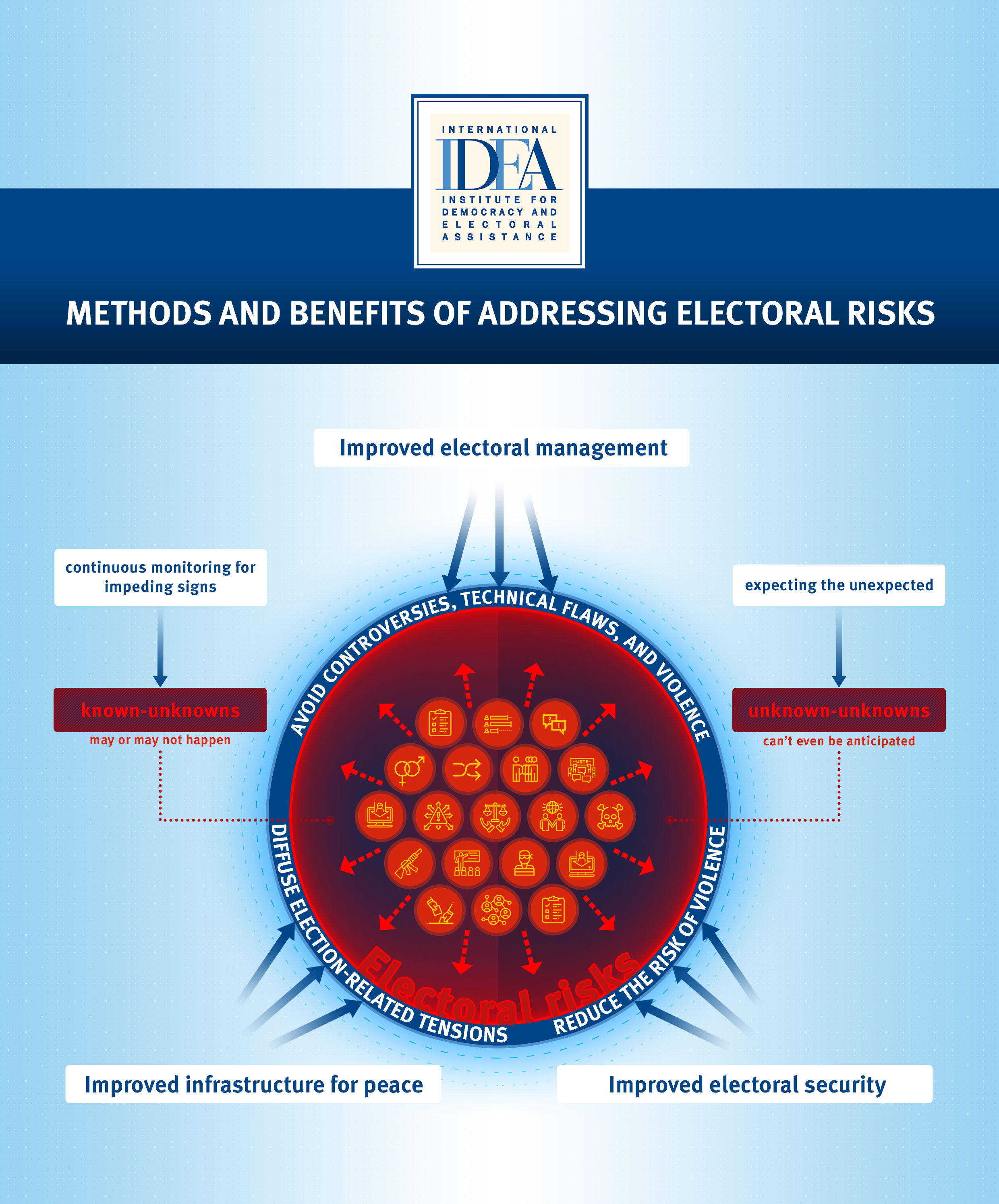 Electoral Risk Management Tool International Idea
Electoral Risk Management Tool International Idea
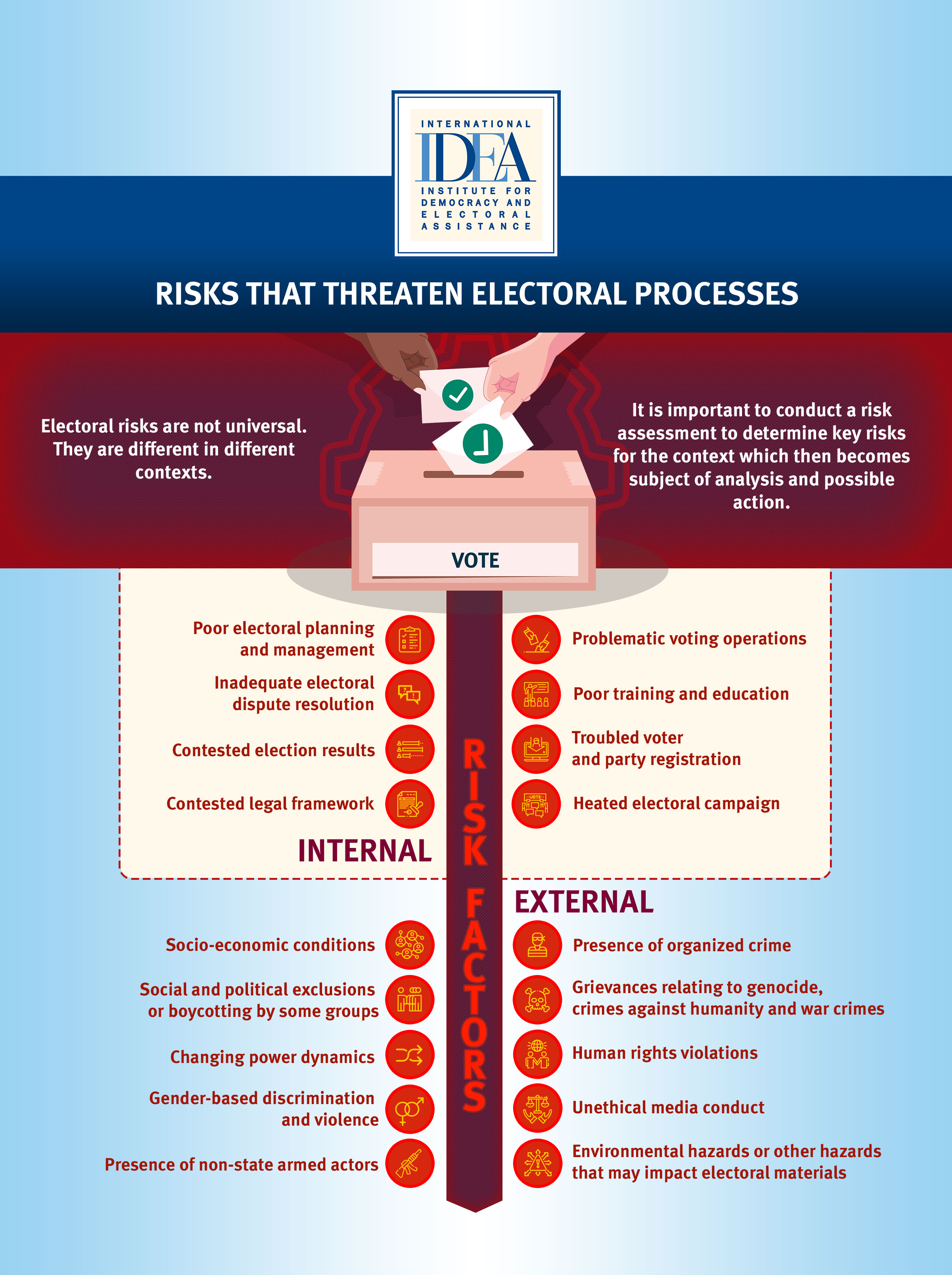 Electoral Risk Management Tool International Idea
Electoral Risk Management Tool International Idea
1 80 01 Enterprise Risk Management Policies And Procedures Library The University Of Queensland Australia
 Risk Management Tools The Mitre Corporation
Risk Management Tools The Mitre Corporation
 Security Risk Management An Overview Sciencedirect Topics
Security Risk Management An Overview Sciencedirect Topics
 Manual Iso 9001 Quality System Manual By Globalmanagergroup Management Templates How To Plan
Manual Iso 9001 Quality System Manual By Globalmanagergroup Management Templates How To Plan
Https Www Theglobalfund Org Media 7540 Financial Financialriskmanagement Guidelines En Pdf U 636784020850000000
 World S Largest Professional Network Cadena De Suministro Cadenas
World S Largest Professional Network Cadena De Suministro Cadenas
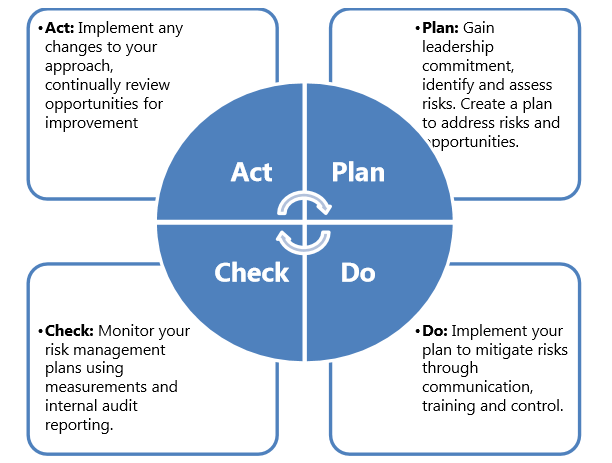 Actions To Address Risks And Opportunities Explained With Procedure
Actions To Address Risks And Opportunities Explained With Procedure
Medical Device Design Risk Management Basic Principles Wipro
 Risk Management Tools The Mitre Corporation
Risk Management Tools The Mitre Corporation
 Firm Business Continuity Planning And Risk Mitigation Strategies Ifac
Firm Business Continuity Planning And Risk Mitigation Strategies Ifac
 Safety Risk Management An Overview Sciencedirect Topics
Safety Risk Management An Overview Sciencedirect Topics
Medical Device Design Risk Management Basic Principles Wipro
Https Www Theglobalfund Org Media 7540 Financial Financialriskmanagement Guidelines En Pdf U 636784020850000000
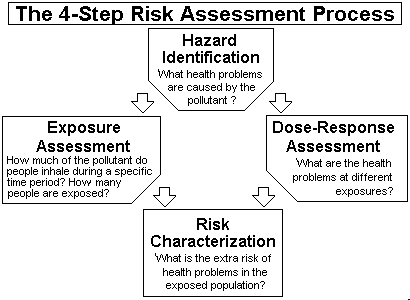 Risk Assessment For Toxic Air Pollutants A Citizen S Guide Technology Transfer Network Air Toxics Web Site Us Epa
Risk Assessment For Toxic Air Pollutants A Citizen S Guide Technology Transfer Network Air Toxics Web Site Us Epa

Post a Comment for "Global Quality & Risk Management Manual"